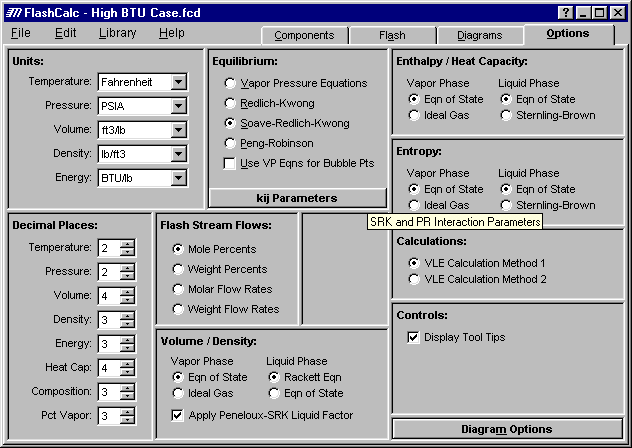
Options - The first step when running a case is to select the desired conditions on the Options page:

The figure above shows the Options tab where the user chooses the vapor-liquid equilibrium model, the physical properties models, and other parameters such as Units and Decimal Places.
Equilibrium - FlashCalc currently offers four different methods for calculating vapor-liquid equilibrium using :
kij Parameters - The program contains common interaction parameters (kij’s) for the SRK and PR equations of state, or the user can enter the parameters if known. The user needs to either select the default values or enter his own values for each case after selecting components. More on kij parameters later in the tour.
Flash Stream Flows - This options controls the format of units in the material balance grid displayed for flash calculations. An example is shown later in this tour on the Properties page discussion.
Calculations - Two proprietary convergence procedures give the maximum possible reliability and range. When solving equilibrium problems two loops must be converged. One loop solves for the phase compositions while the other solves for temperature, pressure or fraction vapor. Typically the composition loop is the outer loop. The user has the option to choose either loops. This allows the program to solve cases involving wide boiling mixtures and cases very close to the critical point, which previously would not converge to a solution.
Volume/Density, Enthalpy/Heat Capacity, and Entropy - The various physical properties can be calculated using an equation of state or one of several user selectable empirical models.
Controls - The Display Tool Tips option activates tips such as the "SRK and PR Interaction Parameters" shown above when the mouse hovers over the kij Parameters button.
Diagram Options - Controls appearance of graphs shown later in the tour.